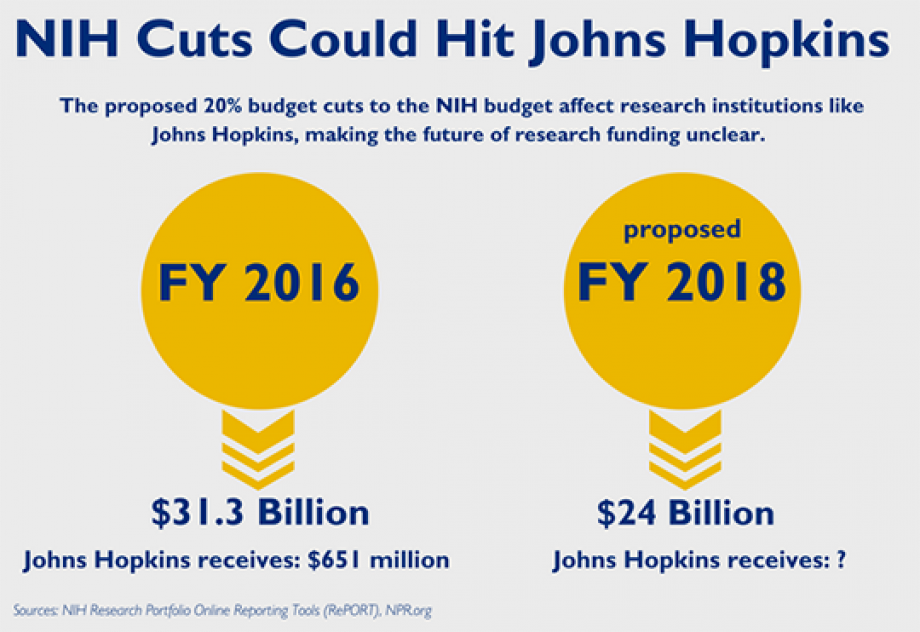
Recent funding cuts in medical research pose a significant threat to patient safety and the integrity of clinical trials across the nation. With federal grants, particularly from the National Institutes of Health (NIH), facing reductions, the resources allocated for oversight and regulatory compliance in studies have sharply declined. This diminishment in medical research funding exacerbates the challenges faced by Institutional Review Boards (IRBs), which play a crucial role in ensuring the welfare of participants. As cutbacks continue, the impact of funding cuts on research quality and safety becomes more pronounced, putting patients at risk and undermining public trust in the medical research process. Consequently, maintaining robust support for research funding is essential to protect both patient safety in research and the development of innovative treatments.
The recent downturn in financial support for clinical research raises critical concerns about the safety and ethical treatment of research participants. Such reductions in resources threaten not only the stability of ongoing studies but also compromise essential oversight mechanisms that safeguard patient welfare. Resource constraints severely limit the ability of regulatory bodies, like IRBs, to conduct thorough reviews and ensure compliance with necessary ethical standards. This situation highlights the importance of adequate funding for research initiatives, which directly correlates with the successful execution of medical studies. As we navigate the complexities of health research funding, it is vital to recognize the role these financial resources play in fostering public confidence and advancing medical progress.
The Consequences of Funding Cuts in Medical Research
Funding cuts in medical research can have devastating effects on various facets of healthcare. When financial resources are slashed, researchers are forced to abandon projects that aim to improve patient outcomes and innovate new treatments. The lack of funding from federal sources, such as NIH funding, directly impacts the ability of institutions to compete for grants and conduct essential studies. As seen with Harvard losing over $2 billion in federal funding, the termination of grants disrupts the collective efforts of numerous research teams, leading to delays in vital medical advancements.
Moreover, cuts in funding disrupt the critical oversight required to maintain patient safety in research. Institutional Review Boards (IRBs), which ensure ethical compliance in clinical trials, rely on financial support to function properly. A diminishment in resources means fewer personnel to manage the heavy influx of proposals that need careful scrutiny. Consequently, this can lead to lapses in participant protections and a systemic increase in ethical violations, ultimately endangering the very individuals these studies seek to help.
The Role of Institutional Review Boards in Patient Safety
Institutional Review Boards (IRBs) play a pivotal role in safeguarding the rights and welfare of patients involved in research. By meticulously reviewing study proposals, IRBs assess risks and benefits, ensure informed consent, and confer ethical oversight throughout the research lifecycle. Their involvement is particularly crucial in multisite studies where coordination between various institutions is required. With the recent stop-work orders stemming from funding cuts, the effective operation of IRBs is jeopardized, raising concerns about whether adequate patient protections can be maintained through these turbulence years for medical research.
The effectiveness of IRBs can suffer when financial constraints limit their ability to hire skilled staff or maintain adequate training programs for existing personnel. Without sufficient resources, IRBs may struggle to fulfill their regulatory responsibilities, such as managing interactions with sponsors and providing guidance to investigators. A well-resourced IRB can offer invaluable support, helping ensure that research adheres to the highest standards of safety and ethics. In contrast, a weakened IRB increases the risk of overlooking critical safety protocols, potentially compromising the integrity of clinical trials.
Regulatory Framework: How Funding Influences Oversight
The regulatory framework governing medical research is deeply intertwined with funding levels. Governmental bodies like the National Institutes of Health (NIH) provide essential resources that facilitate the establishment and maintenance of oversight bodies such as IRBs. When budgets are constricted, the regulations that protect patients can become sluggish, allowing loopholes to emerge. Ultimately, this compromises the integrity of the entire research ecosystem. As history has shown, without stringent oversight, the probability of ethical breaches increases, detrimentally affecting public trust in medical research.
Regulatory measures are designed to evolve alongside scientific advancements, adapting to new ethical dilemmas in clinical research. However, funding cuts can stall this evolution, leaving gaps in the regulatory landscape. As new treatments are developed, the lack of an adaptive regulatory framework can hinder the introduction of innovative therapies that could provide significant health benefits. Therefore, ensuring a continuous flow of resources to regulatory agencies is crucial not only for patient safety but also for the advancement of medical science.
Impact on Collaborative Research and Innovation
Funding cuts also have a pronounced effect on collaborative research initiatives, which are vital for pooling resources and expertise across various institutions. The SMART IRB system represents a significant step forward in reducing administrative burdens for multisite research. However, with federal funding now hindered, over 25 institutions have been unable to participate in ongoing studies, stymying progress on treatments for serious health conditions such as Alzheimer’s disease. The collaborative model that enhances the efficiency and impact of research is at risk of collapse due to these funding freezes.
Innovation in medical research hinges on the ability of investigators from different institutions to work together seamlessly. When funding is constricted, promising projects may face delays or cancellations, curtailing their potential to advance medical knowledge. Without the ability to explore novel therapies and interventions across a network of researchers, the pace of scientific discovery may significantly diminish, prolonging the suffering of patients who could benefit from new treatments. Collaborative networks must be supported with adequate funding to foster an environment ripe for innovation.
Restoring Trust Through Ethical Oversight
Trust is fundamental to the success of medical research, particularly when it comes to patient participation. The recent disruptions caused by funding cuts generate skepticism about the motives of researchers and the safety of experimental procedures. IRBs function as the guardians of this trust, providing assurances that ethical considerations are paramount in research practices. When funding cuts limit their ability to operate fully, public confidence in research can wane, hindering recruitment for clinical trials and ultimately affecting medical advancements.
Rebuilding this trust following funding disruptions calls for transparent communication between institutions and the public. Clear narratives about the role of IRBs and how they protect participant safety are necessary to reassure patients and communities. Efforts to restore confidence must prioritize the ethical conduct of research, ensuring that oversight mechanisms are not underfunded and that researchers remain accountable. As we navigate these challenging times, a recommitment to ethical practices in medical research is essential for maintaining public trust.
Historical Context: Lessons from Past Research Abuses
Historical events offer critical lessons regarding the necessity for strict oversight in medical research, especially in the wake of funding cuts. The infamous Tuskegee Syphilis Study and other ethical violations underscore the ongoing importance of IRBs in safeguarding research participants. Regulatory frameworks that emerged from these historical injustices highlight the critical need for a well-financed system that can rigorously enforce ethical standards in research. When funding is slashed, it not only impacts current studies but risks reverting to a past where participant protection was often overlooked.
The responsibility to learn from history is ever-present, particularly in ensuring that research practices evolve alongside societal expectations. Maintaining a robust IRB framework funded adequately is essential to prevent heinous abuses from reoccurring. These oversight boards are not only responsible for safeguarding current participants but also for fostering an environment of ethical responsibility that future researchers must abide by. Learning from the past compels us to ensure that funding for medical research is not compromised, enabling those who conduct studies to prioritize patient safety and ethical conduct.
The Challenge of Balancing Financial Constraints and Research Needs
Balancing financial constraints with the pressing needs of medical research presents a significant challenge. As funding cuts continue, research institutions must prioritize which projects to support, often resulting in difficult decisions about which potential breakthroughs may never see the light of day. The dwindling pool of available resources forces research teams to make trade-offs that can compromise the quality and comprehensiveness of studies. This scenario is not only detrimental to researchers but also to the health outcomes of patients awaiting new therapies.
In addition to prioritizing projects, researchers must also grapple with the increasing administrative burden that limited funding imposes. The complexity of navigating reduced financial support creates obstacles that detract from the core focus of innovation in patient care. Researchers find themselves spending more time on securing funding and documenting compliance with regulatory requirements instead of advancing their scientific inquiries. The ecosystem of medical research requires a sustained commitment to appropriate funding levels to foster innovation, not hinder it.
Community Engagement: Involving the Public in Research Funding Decisions
Engaging the public in discussions around research funding is increasingly important, especially in light of recent funding cuts. When communities are involved in the decision-making process regarding research priorities, the outcomes are typically more aligned with public health needs and ethical considerations. Involvement in funding decisions fosters a sense of shared responsibility, ultimately enhancing trust between researchers and the communities they serve. This collaborative spirit strengthens the ethical underpinnings of medical research and can mitigate public skepticism.
To cultivate ongoing community engagement, research institutions must prioritize transparency in their operations and funding allocations. Open forums and community consultations can provide a platform for shared dialogue, allowing researchers to explain the importance of their work and how funding decisions directly impact public health. When individuals feel invested in the research process, they are more likely to support efforts to maintain adequate funding levels, bolstering the integrity and efficacy of research endeavors.
Frequently Asked Questions
What are the consequences of funding cuts in medical research on patient safety?
Funding cuts in medical research significantly jeopardize patient safety as they disrupt vital oversight processes managed by Institutional Review Boards (IRBs). Without adequate funding, IRBs may face staffing shortages, reducing their ability to monitor research compliance and ensure patient welfare rigorously. This can lead to a lack of thorough reviews of clinical trials, ultimately putting participants at risk.
How do funding cuts in medical research affect NIH funding for patient safety initiatives?
The reduction in funding for medical research has a detrimental impact on NIH funding, which is crucial for initiatives that enhance patient safety. NIH funds are instrumental in supporting the IRB review processes that safeguard participants’ rights and welfare in clinical studies. Cuts can result in decreased resources for these protective measures, fostering an environment where potential risks to patients may go unchecked.
What is the impact of funding cuts in medical research on collaborative studies and multi-site research oversight?
Funding cuts in medical research severely impede collaborative studies and multi-site research oversight. Essential programs like the SMART IRB, which streamline the review process across institutions, may be halted, causing delays in research and limiting the ability to add new sites. This disruption compromises the momentum of scientific discoveries that could benefit patient care.
How do funding cuts affect insurance of ethical practices in medical research?
Funding cuts in medical research directly impact the ability of IRBs to uphold ethical practices. These boards depend on adequate resources to perform detailed evaluations of studies, ensuring informed consent and protection of vulnerable populations. A decrease in funding can lead to oversights in ethical regulations, undermining the integrity of research and the trust of participants.
What role does NIH funding play in mitigating the impact of funding cuts in medical research?
NIH funding plays a pivotal role in mitigating the impact of funding cuts in medical research by providing essential financial resources for patient safety programs and ethical oversight through IRBs. It supports the training, operation, and compliance monitoring critical to ensuring safe research practices and participant well-being, which become even more crucial during times of restricted funding.
In what ways can patient safety be compromised due to the impact of funding cuts in medical research?
Patient safety can be compromised due to the impact of funding cuts in medical research through increased risks in clinical trials. This includes insufficient monitoring of adverse events, inadequate participant support and education, and possible delays in the implementation of safety protocols. Consequently, such disruptions can foster an environment where patient rights are at greater risk.
What actions can institutions take in response to funding cuts in medical research to maintain patient safety?
In response to funding cuts in medical research, institutions can seek alternative funding sources, allocate existing resources strategically, and strengthen partnerships with private organizations. Additionally, advocates can lobby for increased governmental support for medical research funding to safeguard patient safety and enhance IRB capabilities to monitor and protect research participants.
| Key Points | Details |
|---|---|
| Funding Cuts Impact | The Trump administration froze over $2 billion in federal research funds to Harvard, disrupting vital medical research efforts. |
| Role of IRBs | Institutional Review Boards (IRBs) oversee research studies to protect participant rights and ensure compliance with regulations. |
| Significance of SMART IRB | SMART IRB facilitates oversight for collaborative research at multiple sites, important for studies involving human participants. |
| Consequences of Halted Studies | Halting studies due to funding issues can lead to delays, increased risks to participants, and decreased public trust in research. |
| Historical Context | Historical abuses in medical research led to the establishment of IRBs to ensure ethical standards and participant protection. |
| Future of Research | Cutbacks in funding will hinder the ability of researchers to innovate and collaborate effectively, impacting public health. |
Summary
Funding cuts in medical research severely undermine efforts aimed at protecting patient safety and ensuring the integrity of research studies. The recent freeze on federal research funding to Harvard has disrupted critical oversight mechanisms managed by IRBs, which are essential in safeguarding participants’ rights and wellbeing. Without adequate funding, ongoing studies face potential halts, exacerbating public skepticism and mistrust in the research process, which can have long-lasting effects on scientific advancement and health outcomes.