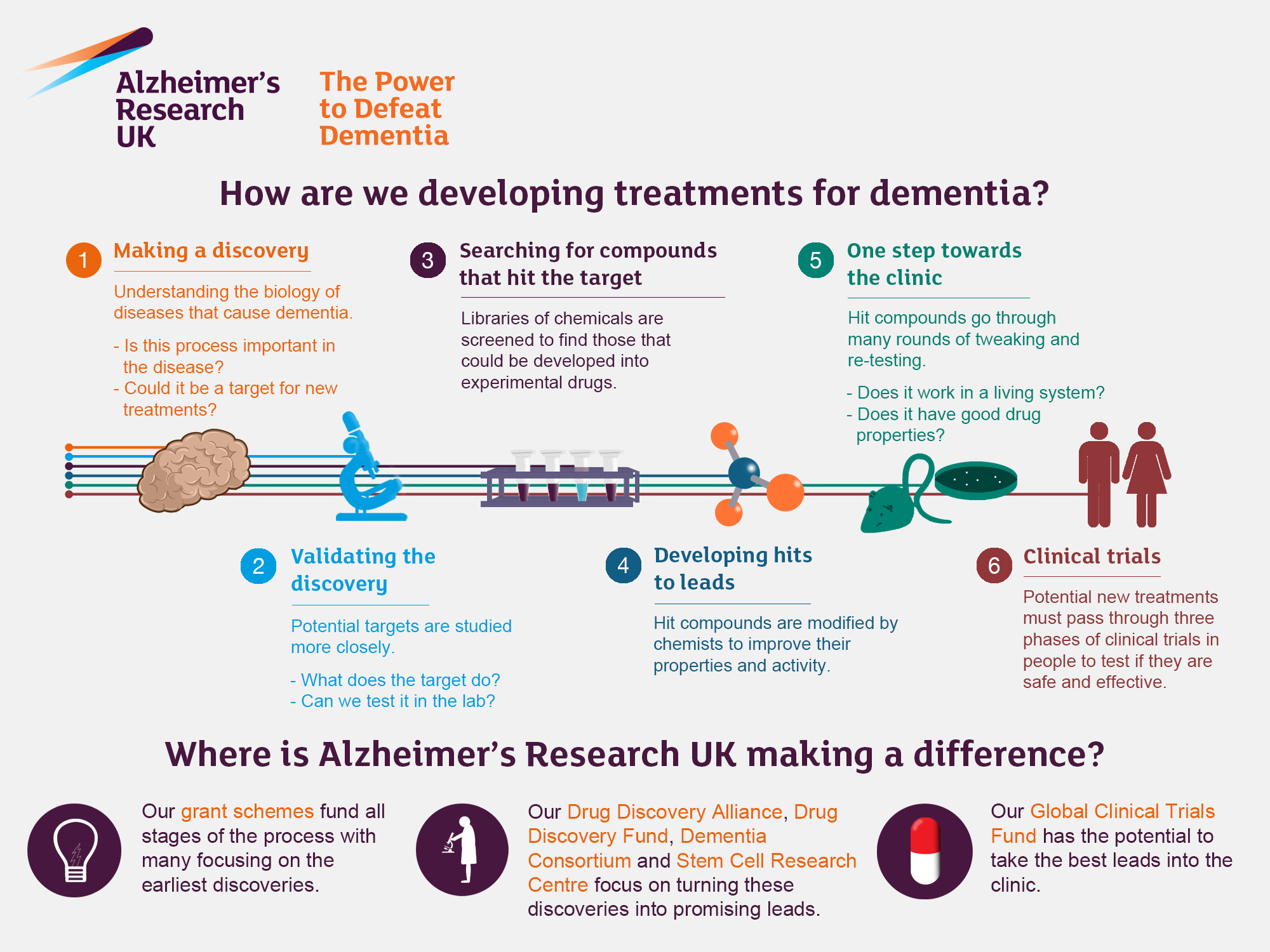
Alzheimer’s research is at the forefront of understanding one of the most challenging neurodegenerative diseases affecting millions worldwide. Pioneered by scientists like Beth Stevens, this field delves into the intricate roles of microglial cells in the brain’s immune system, which are crucial for maintaining neurological health. These cells are not just guardians; they actively participate in synaptic pruning—removing unnecessary connections between neurons to streamline communication. However, when this process goes awry, it can contribute to the onset of conditions like Alzheimer’s disease, highlighting the need for innovative biomarkers for Alzheimer’s to track disease progression. With ongoing studies and findings emerging from esteemed institutions, the quest to unravel the complexities of Alzheimer’s is not just about understanding disease, but also about paving the path for new treatments that could alter the course of this debilitating condition.
Investigating the complexities of Alzheimer’s disease has led researchers to uncover vital insights that extend beyond traditional concepts of cognitive decline. This area of study incorporates various terminologies, including study of cognitive degeneration, the roles of the brain’s immune agents, and mechanisms of cellular remodeling. Central to this exploration are microglial cells, which function as the brain’s defense system, ensuring that the intricate web of neuronal connections is preserved through a balanced process of synaptic refinement. The identification of reliable biomarkers for Alzheimer’s is also a crucial step in this journey, facilitating early diagnosis and therapeutic interventions. As scientists like Beth Stevens continue to investigate these fascinating elements, the potential for breakthroughs in preventing or treating neurodegenerative conditions becomes increasingly optimistic.
Understanding Alzheimer’s Disease and Neurodegenerative Disorders
Alzheimer’s disease represents one of the most prevalent neurodegenerative disorders affecting millions worldwide. Characterized by progressive memory loss, cognitive decline, and eventually the inability to perform daily activities, this condition poses significant challenges not just for those diagnosed, but also for caregivers and healthcare systems. Research into Alzheimer’s has shifted to focus on understanding the underlying biological mechanisms that drive disease progression, including the role of neuroinflammation, synaptic dysfunction, and the impact of genetic and environmental factors.
Additionally, researchers are identifying critical biomarkers for Alzheimer’s that may aid in earlier diagnosis and targeted therapies. These biomarkers include proteins like amyloid-beta and tau, which accumulate in the brains of affected individuals. As we delve deeper into these biological markers, novel therapeutic strategies can be developed, which may one day lead to effective treatments to combat this relentless disease.
The Role of Microglial Cells in Alzheimer’s Research
Microglial cells serve an essential function in maintaining brain health by acting as the primary immune defense in the central nervous system. These cells continuously monitor the brain environment, responding to injury and disease. In the context of Alzheimer’s disease, microglia play a pivotal role in synaptic pruning—a process critical for neurodevelopment but when dysregulated, may lead to the loss of synapses and cognitive function. Recent studies in Beth Stevens’ lab have highlighted how microglial dysfunction contributes to the pathology of Alzheimer’s, opening new avenues for therapeutic intervention.
Stevens’ research has underscored that proper microglial function is vital for clearing out excess amyloid plaques and maintaining synaptic integrity. However, when microglial cells become overactive or malfunction, they may instead promote neuroinflammation and exacerbate neurodegeneration. Understanding these dynamics is crucial for developing strategies aimed at harnessing microglial activities to prevent or slow down Alzheimer’s progression.
Significance of Synaptic Pruning in Neurodegenerative Diseases
Synaptic pruning, a natural and necessary process wherein excess synaptic connections are eliminated, is crucial during brain development and learning. However, in neurodegenerative conditions such as Alzheimer’s, aberrant pruning can occur, leading to synaptic loss and impaired cognitive function. Beth Stevens’ groundbreaking work has shed light on how disruptions in the pruning process performed by microglial cells can be linked to increased risk of Alzheimer’s disease, with significant implications for both understanding and treating these conditions.
The realization that synaptic pruning is not a mere cleanup crew, but an active participant in shaping neural networks, presents new opportunities for interventions. By targeting the mechanisms underlying dysregulated synaptic pruning, researchers hope to develop innovative therapies that enhance synaptic health and potentially reverse cognitive decline. This research not only illuminates the pathophysiology of Alzheimer’s but also emphasizes the need for a more integrated approach to tackling various neurodegenerative diseases.
Innovative Biomarkers for Alzheimer’s Disease Detection
The development of reliable biomarkers for Alzheimer’s disease is a crucial step towards improving diagnosis and treatment strategies. Biomarkers like amyloid plaques and tau tangles have traditionally been used to confirm Alzheimer’s post-mortem; however, advancements in imaging techniques and blood-based biomarkers are paving the way for earlier diagnosis. Research conducted in labs like Beth Stevens’ is exploring new avenues for identifying these molecular signatures which can be detected in living patients.
Recognizing these biomarkers can significantly improve patient management by enabling clinicians to personalize treatments while monitoring disease progression. Additionally, these findings can spur further research into novel therapeutic approaches that target the precise moments of pathology rather than waiting for clinical symptoms to manifest. Ultimately, enhancing our understanding of Alzheimer’s biomarkers is pivotal for innovative treatments and improving the quality of life for those affected.
Beth Stevens’ Contributions to Alzheimer’s Research
Beth Stevens has emerged as a prominent figure in Alzheimer’s research, making substantial contributions that have reshaped our understanding of the disease. With her pioneering studies on microglial cells, she has illuminated the mechanisms through which these immune cells interact with neurons, particularly in the context of synaptic pruning and neurodegeneration. Stevens’ work demonstrates that the brain’s immune response plays a critical role in the progression of Alzheimer’s disease.
Moreover, Beth Stevens’ innovative approach emphasizes the importance of curiosity-driven research in scientific discovery. By investigating basic questions about microglial function, she has inadvertently laid the groundwork for therapeutic strategies that could one day alter the course of Alzheimer’s disease. Schisms between basic science and clinical application have bridged under her leadership, promoting a more holistic understanding of neurodegenerative diseases.
The Impact of Federal Funding on Alzheimer’s Studies
Federal funding has been instrumental in advancing Alzheimer’s research, providing the necessary resources for scientists like Beth Stevens to explore groundbreaking concepts in neurobiology. Much of this funding comes from the National Institutes of Health (NIH), which has prioritized research on aging and neurodegenerative diseases, allowing laboratories to pursue innovative studies without the restrictions commonly found in private funding.
As Stevens describes, this stable financial backing during the formative years of her research lab was essential for fostering a culture of exploration and discovery. The support not only enables studies on microglial functions but also promotes interdisciplinary collaboration, which is critical for tackling complex diseases such as Alzheimer’s. Continued investment in research will be fundamental for uncovering new insights and developing effective interventions.
Exploring Neuroinflammation in Neurodegenerative Diseases
Neuroinflammation is increasingly recognized as a central player in the pathophysiology of neurodegenerative diseases, including Alzheimer’s. The activation of microglial cells and the release of inflammatory cytokines can influence neuronal health and synaptic function, leading to further neurodegeneration. Understanding the complexities of neuroinflammation through research like that of Beth Stevens is essential for creating targeted therapies that may mitigate these harmful processes.
In Stevens’ lab, researchers are investigating how to modulate the inflammatory response of microglial cells to protect synapses and promote cognitive function. By harnessing the immune properties of microglia, there lies potential for novel therapeutic approaches that could fundamentally change how Alzheimer’s disease is treated, targeting the underlying inflammation rather than just symptomatic relief. Addressing neuroinflammation paves the way for more effective management of Alzheimer’s and other related disorders.
Future Directions in Alzheimer’s Disease Research
The future of Alzheimer’s disease research holds exciting prospects as scientists continue to deepen their understanding of complex brain functions and interactions. Areas of current exploration include the relationship between synaptic health and cognitive decline, the role of genetics in susceptibility to Alzheimer’s, and the therapeutic potentials of targeting specific biomarkers. As researchers build on foundational studies from pioneers like Beth Stevens, a more comprehensive picture of Alzheimer’s disease will emerge.
Additionally, with advances in technology such as neuroimaging and genomic editing, researchers have unprecedented tools to explore the intricacies of neurodegenerative diseases. Exciting developments in this field may not only enhance diagnosis and treatment options but also lead to preventive strategies that could fundamentally alter the trajectory of Alzheimer’s disease for future generations. Collaborative efforts among scientists, clinicians, and funding agencies will be crucial in pushing the boundaries of what is currently known.
The Intersection of Basic Science and Clinical Application in Alzheimer’s Research
Basic scientific research lays the groundwork for clinical applications, particularly in the field of Alzheimer’s disease. Researchers like Beth Stevens demonstrate that understanding the underlying biology of microglial cells and synaptic mechanisms is essential for developing effective treatments. Basic science provides the insights necessary for researchers to formulate hypotheses regarding disease processes and potential therapeutic targets.
Translational research bridges the gap between bench and bedside, transforming discoveries into tangible health solutions. By fostering interdisciplinary collaborations and emphasizing the importance of basic research, scientists can create a continuum of knowledge that leads to novel therapies for Alzheimer’s disease. Addressing complex biological questions ultimately illuminates pathways that can improve the lives of those suffering from neurodegenerative disorders.
Frequently Asked Questions
What role do microglial cells play in Alzheimer’s research?
Microglial cells are crucial in Alzheimer’s research as they act as the brain’s immune system. They patrol for signs of damage and help prune synapses, processes that can become detrimental in conditions like Alzheimer’s disease. Aberrant synaptic pruning by microglia has been linked to neurodegenerative diseases, making them a focal point in studies aimed at understanding and developing treatments for Alzheimer’s.
How do biomarkers for Alzheimer’s disease aid in research?
Biomarkers for Alzheimer’s play an essential role in research by allowing scientists to detect the disease earlier and monitor its progression. They are often linked to the pathological processes associated with neurodegenerative diseases, including the activity of microglial cells and changes in synaptic health, which can lead to the development of targeted therapies.
What is synaptic pruning, and why is it significant in Alzheimer’s research?
Synaptic pruning is the process by which excess synapses are eliminated to enhance the efficiency of neuronal networks. In Alzheimer’s research, understanding how microglial cells participate in pruning is vital because improper pruning can contribute to the neurodegenerative processes observed in the disease.
How has Beth Stevens influenced the study of Alzheimer’s disease?
Beth Stevens has significantly influenced Alzheimer’s research by transforming our understanding of microglial cells and their role in synaptic pruning. Her work highlights how aberrant microglial activity can lead to Alzheimer’s and other neurodegenerative diseases. This research lays the groundwork for identifying new biomarkers and therapies aimed at treating Alzheimer’s.
Why is studying microglial cells important for neurodegenerative disease research?
Studying microglial cells is important because they provide insights into the brain’s immune responses and their impact on neurodegenerative diseases like Alzheimer’s. By understanding how microglia interact with synapses and other cells in the brain, researchers can identify mechanisms that lead to disease and develop effective interventions.
What breakthroughs in Alzheimer’s research have come from understanding microglial function?
Breakthroughs in Alzheimer’s research have emerged from understanding microglial function, particularly their role in synaptic pruning. Studies have shown that dysfunctional microglial activity can exacerbate neurodegeneration, guiding researchers towards potential biomarkers and new therapeutic strategies to combat Alzheimer’s disease.
How does the research on microglial cells contribute to the understanding of cognitive decline in Alzheimer’s patients?
Research on microglial cells contributes to understanding cognitive decline in Alzheimer’s patients by revealing how these immune cells regulate neuronal health and connectivity. Aberrant pruning and inflammatory responses from microglia can disrupt synaptic function, leading to cognitive deficits characteristic of Alzheimer’s.
What funding sources support Alzheimer’s research focused on microglial cells?
Funding for Alzheimer’s research focused on microglial cells often comes from federal agencies such as the National Institutes of Health (NIH). This financial support is crucial for exploring basic science questions that drive advancements in understanding neurodegenerative diseases like Alzheimer’s.
| Key Point | Details |
|---|---|
| Microglial Function | Microglia serve as the brain’s immune system, clearing out dead cells and pruning synapses. |
| Role in Alzheimer’s Research | Aberrant pruning by microglia may contribute to neurodegenerative diseases including Alzheimer’s. |
| Impact of Research | Research led by Beth Stevens forms a foundation for new biomarkers and medicines for treating Alzheimer’s. |
| Funding and Support | Beth Stevens’ work was largely supported by federal funding from the NIH. |
| Scientific Curiosity | Basic science research can lead to significant breakthroughs in understanding and treating diseases. |
Summary
Alzheimer’s research is at a pivotal point, with groundbreaking insights into the role of microglial cells in the brain’s immune response. This research not only deepens our understanding of Alzheimer’s disease but also opens pathways for the development of new treatments and biomarkers. As the scientific community continues to investigate these mechanisms, the potential for improved care for the millions affected by Alzheimer’s grows, underscoring the importance of ongoing funding and curiosity-driven inquiry in the field.